PETALING JAYA: Thomson Hospital Kota Damansara (THKD), the flagship hospital of TMC Life Sciences Berhad (TMCLS), today (July 12) became the first hospital in Southeast Asia to administer Spinraza, a US Food and Drug Administration (FDA)-approved treatment for Spinal Muscular Atrophy (SMA).
The recipient of the treatment is a 5-year-old Sarawakian child, who has been diagnosed with SMA since birth. This first initiative is part of THKD’s collaborative Corporate Social Responsibility (CSR) program with One Hope Charity & Welfare to provide treatment for the period of 2 years. The procedure was performed by Consultant Paediatric Anaesthesiologist Dr Foo Sze Yuen in the presence of Consultant Paediatrician & Paediatric Neurologist Dr Sangita Dharshini Terumalay.
THKD chief executive officer Nadiah Wan said THKD is proud to be at the forefront of this advancement in paediatric care. As part of TMC Life Sciences Berhad, a leading women & children’s healthcare group, THKD is dedicated to achieving new milestones in paediatric and women’s health, as demonstrated in our commitment as a participating hospital in the MySpin Patient Assistance Programme for SMA patients.
“The administration of intrathecal Spinraza is a testament to our pursuit of excellence, in line with our focus on women & children services as we move into more paediatric sub-specialties. With Spinraza, we can significantly improve the quality of life for children battling SMA. Its introduction into our treatment protocols empowers us to deliver even more targeted and effective care, elevating the standard of paediatric medicine and instilling hope in families facing this challenging genetic disorder. It is also a manifestation of our vision of helping people lead healthier lives by making healthcare accessible, accountable and sustainable.”
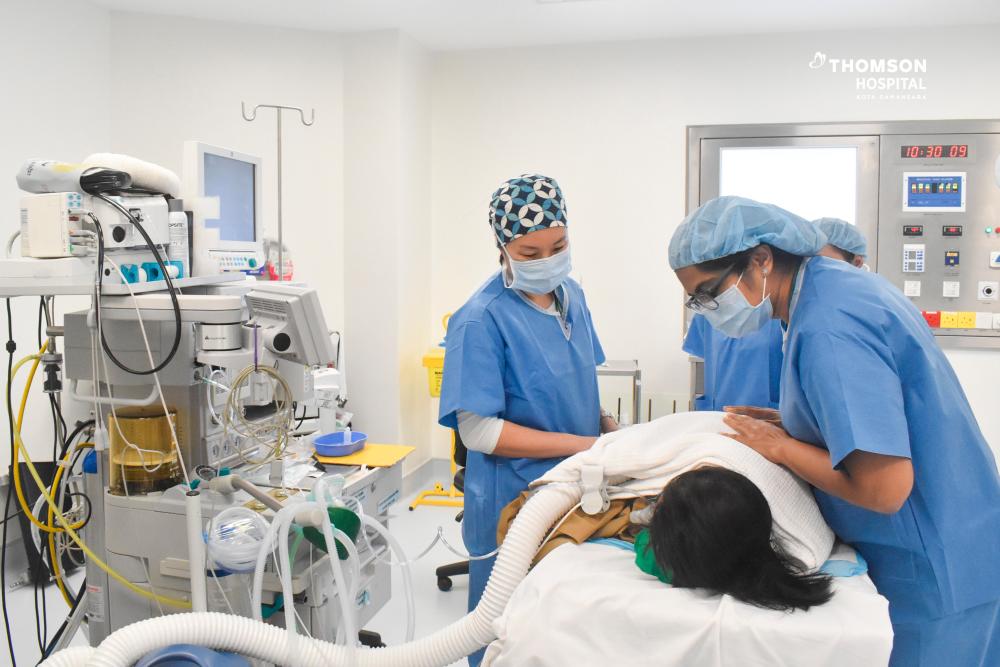
Dr Sangita said the administering of Spinraza in the hospital ushers in a new era of hope and enhanced care for children. “My primary objective as a paediatrician is to ensure the well-being and recovery of every child entrusted to our care. This certainly represents a milestone in paediatric medicine, offering an effective treatment that will positively impact the lives of affected children.”
Spinraza received US FDA approval in 2016 after a series of trials that showed meaningful benefits in seven clinical trials involving infants and children with SMA, thus becoming the first approved therapy for paediatric and adult patients with SMA.
According to the National Institute of Neurological Disorders and Stroke, Spinraza is administered via injection into the fluid surrounding the spinal cord by a physician with SMA experience. Spinraza works as a disease modifying treatment that targets the underlying cause of muscle weakness in SMA – the Survival Motor Neuron (SMN) protein.
SMA is an inherited neurological genetic disorder that affects the central nervous system causing the nerve cells in the spinal cord and brainstem not to work properly. It impacts individuals across a range of ages, from infants and children to teens and adults, and with varying levels of severity.
It is estimated that one in every 10,000 births worldwide is diagnosed with SMA, an inherited disease, and around one in every 40-50 people globally are carriers of the mutated gene.
To find out more about paediatric services in THKD, visit https://thomsonhospitals.com.
*K.K.L.I.U.: 1442/EXP: 31.12.2023









