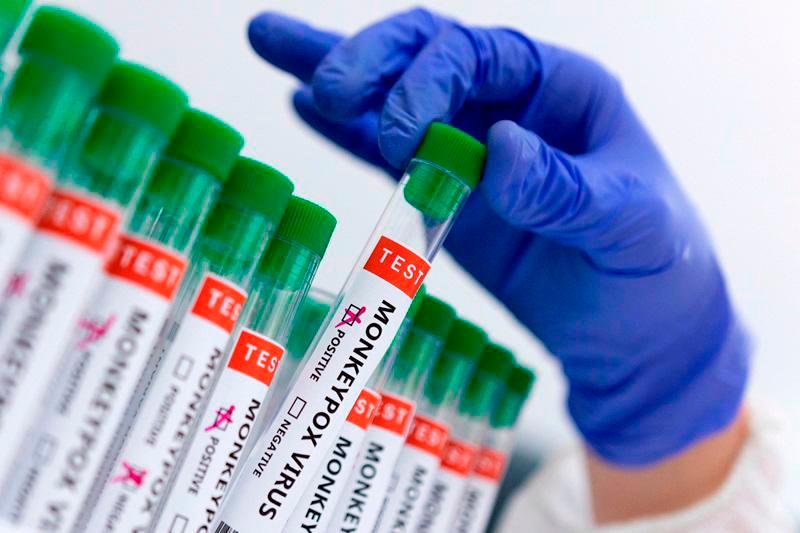
KUALA LUMPUR: The Medical Device Authority (MDA) has approved the first monkeypox (mpox) test kit for the Malaysian market to aid in the effective detection of the virus.
MDA chief executive Dr Muralitharan Paramasua, however, said the Viasure Monkeypox Virus Real-Time PCR Detection Kit can only be handled by authorised professionals, such as licensed medical practitioners, and not by the general public.
He said that selling or purchasing the kit in pharmacies, retail stores, or e-commerce platforms is prohibited and is an offence under the Medical Device Act 2012 (Act 737).
“If the mpox test kit is not handled by professionals, there is a possibility of inaccurate results and may cause the virus to spread among the public.
“The public is advised not to purchase or use any monkeypox (mpox) test kit on their own,” he said in a statement today.
Dr Muralitharan urged anyone who encounters the kit being sold to the public in pharmacies, retail stores, or on e-commerce platforms to report it to the authorities via the MDA feedback management system at femes.mda.gov.my.
According to the Ministry of Health (MOH), 33 suspected mpox cases have been reported at health facilities, with 32 cases confirmed as negative and one case still awaiting laboratory results as of yesterday.
MOH also said that 13 laboratories were equipped to conduct mpox detection tests, comprising eight government labs and five private labs, two of which are capable of genetic sequencing to detect the virus variants.